![]() Más información sobre la EPI/neumonitis
Más información sobre la EPI/neumonitis
*Esta información es específica para profesionales sanitarios.
*Esta información es específica para profesionales sanitarios.
![]() Más información EPI/neumonitis
Más información EPI/neumonitis
Factores de riesgo generales vinculados a la enfermedad pulmonar intersticial/neumonitis relacionadas con otros Medicamento
Aun no se conocen los mecanismos exactos por los cuales ENHERTU® (trastuzumab deruxtecán) puede causar enfermedad pulmonar intersticial.6
Los factores de riesgo generales para el desarrollo de enfermedad pulmonar intersticial inducida por medicamentos varían según la enfermedad, el medicamento y la población considerada, e incluyen los siguientes:7,8,9
- Antecedentes de enfermedad pulmonar intersticial o enfermedad pulmonar: la enfermedad pulmonar preexistente y la función pulmonar reducida son factores de riesgo importantes de la enfermedad pulmonar intersticial inducida por medicamentos.7,9,10,11
- Estado de salud deficiente: en oncología, un estado funcional deficiente o una enfermedad metastásica pueden aumentar el riesgo de presentar enfermedad pulmonar intersticial inducida por medicamentos.8
- Tabaquismo: los fumadores corren un mayor riesgo de presentar enfermedad pulmonar intersticial inducida por medicamentos.7
- Edad avanzada: las personas de edad avanzada, especialmente los mayores de 60 años, pueden tener un riesgo significativamente mayor de presentar enfermedad pulmonar intersticial inducida por medicamentos.7,9,11
- Etnia: los pacientes japoneses o afroamericanos pueden correr un mayor riesgo de presentar enfermedad pulmonar intersticial inducida por medicamentos.9,12
- Sexo masculino: los hombres pueden correr un mayor riesgo de presentar enfermedad pulmonar intersticial inducida por medicamentos.7,11
- Tratamiento previo: la quimioterapia previa, el tratamiento con múltiples regímenes de quimioterapia, la radioterapia torácica y la terapia combinada con múltiples agentes dirigidos a moléculas, con o sin citotóxicos, pueden aumentar el riesgo de un paciente de presentar enfermedad pulmonar intersticial inducida por medicamentos.7,8,9
Instrucciones para el manejo de la enfermedad pulmonar intersticial/neumonitis
- Se debe tratar de inmediato cualquier indicio de enfermedad pulmonar intersticial/neumonitis con objeto de detener la inflamación y evitar una fibrosis irreversible con un desenlace potencialmente mortal. 9
| Grado CTCAE | Descripción | Modificación del tratamiento | |||||||||||||||
| Grado 1 | Asintomáticas; | Interrumpir ENHERTU® (trastuzumab deruxtecán) hasta que el acontecimiento remita a grado 0, luego:
|
|||||||||||||||
| Grado 2 o mayor | Sintomáticas; | Suspender permanentemente el tratamiento con ENHERTU® (trastuzumab deruxtecán).
|
Clasificación basada en los Criterios de Terminología Común para Acontecimientos Adversos del Instituto Nacional del Cáncer (NCI CTCAE).13
Referencias
Kubo K, Azuma A, Kanazawa M, et al; Comité de la Sociedad Respiratoria Japonesa. Declaración de consenso para el diagnóstico y el tratamiento de lesiones pulmonares inducidas por fármacos. Respir Invest. 2013;51(4):260-77.
Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-21.
Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097-5108. 7
Skeoch S, Weatherley N, Swift AJ, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7(10):356.
Yonemori K, Hirakawa A, Kawachi A, et al. Drug induced interstitial lung disease in oncology phase I trials. Cancer Sci. 2016;107(12):1830-1836.
Schwaiblmair M, Behr W, Haeckel T, et al. Drug induced interstitial lung disease. Open Respir Med J. 2012; 6:63-74.
Sakurada T, Kakiuchi S, Tajima S, et al. Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother. 2015;49(4):398-404.
Osawa M, Kudoh S, Sakai F, et al. Clinical features and risk factors of panitumumab-induced interstitial lung disease: a postmarketing all-case surveillance study. Int J Clin Oncol. 2015;20(6):1063-71.
Vansteenkiste J. Nivolumab for NSCLC in Japanese patients: similar benefits, but beware of pneumonitis. ESMO Open. 2017;2(suppl 1):e000119.
Departamento de Salud y Servicios Sociales de los Estados Unidos. Criterios terminológicos comunes para la evaluación de reacciones adversas (CTCAE), Versión 5.0 Publicados el 27 de noviembre de 2017.
Más información sobre la EPI/neumonitis
The exact mechanisms via which Enhertu may cause ILD are not yet known3.
General risk factors for the development of drug-induced ILD vary according to the disease, drug, and population being considered and include the following4,5,6
Pre-existing lung disease or reduced lung function5,6,7,8
Poor performance status or metastatic disease5
Smoking4
Advanced age: especially those over 60 years old4,6,8
Japanese or African American Ethnicity6,9
Male sex4,8
Prior treatment: chemotherapy, thoracic radiotherapy, and combination therapy with multiple molecular targeted agents with or without cytotoxic agents4,5,6
Patients should be managed with the goal of suppressing inflammation and preventing irreversible fibrosis with potential fatal outcome.
| CTCAE Grade | Description | Treatment Modification |
| Grade 1 | Asymptomatic; Clinical or diagnostic observations only; Intervention not indicated | Interrupt Enhertu until the event resolves to Grade 0 then:
|
| Grade 2 | Symptomatic; medical intervention indicated; limiting instrumental activities of daily living | Permanently discontinue Enhertu
|
| Grade 3 | Severe symptoms; limiting self care activities of daily living; oxygen indicated | |
| Grade 4 | Life-threatening respiratory compromise; urgent intervention indicated (e.g. tracheotomy or intubation) |
Grading based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events(CTCAE)10
Familiarise yourself with the Enhertu Summary of product Characteristics (SmPc)
Refer to Enhertu and trastuzumab deruxtecan when discussing the drug with the patient
Ensure Enhertu and trastuzumab deruxtecan are written on the prescription and in patient notes, do not abbreviate, truncate or omit any name
Check the correct medication is identified in electronic systems before selecting, potential areas for confusion:
Ensure the medication prescribed is Enhertu (trastuzumab deruxtecan) and not trastuzumab or trastuzumab emtansine
Alphabetical name sorting
Trastuzumab, Trastuzumab emtansine and Trastuzumab deruxtecan may be positioned one after the other
| Medication | Strength |
| Trastu | |
| Trastuzumab | |
| Trastuzumab deruxtecan | 100mg |
| Trastuzumab emtansine | 100mg |
Name truncation
If the system only displays part of the medication name in its drop-down menu or text window (e.g. trastuzumab, trastuzumab deruxtecan or trastuzumab emtansine)
| Medication | Strength |
| Trastu | 100mg |
| Trastuzuma | 150mg |
| Trastuzuma | 100mg |
| Trastuzuma | 160mg |
Limited text field
If the system only displays part of the medication name in its drop-down menu or text window (e.g. trastuzumab, trastuzumab deruxtecan and trastuzumab emtansine)
| Medication | Strength |
| Trastu | 100mg |
Ensure the correct medication is clearly recorded in the patient history
Familiarise yourself with the Enhertu Summary of product Characteristics (SmPc)
Check vial labels, including colour of labels, to ensure that the medicinal product being prepared and administered is ENHERTU® (trastuzumab deruxtecan) and not a trastuzumab-containing products such as Herceptin® (trastuzumab) or Kadcyla®(trastuzumab emtansine).
Check that protocols to avoid medication errors are in place at the hospital/site and that they are followed
Be aware when reading prescriptions that there are multiple types of medication with a similar INN (e.g. trastuzumab, trastuzumab SC, trastuzumab emtansine and trastuzumab deruxtecan)
Double check the intended medication is ENHERTU® (trastuzumab deruxtecan) and that both are entered in the prescription and/or medical history
In case of any doubt, consult with the treating physician
Familiarise yourself with the different cartons, labels and cap colours available for all trastuzumab containing products to select the correct carton
Ensure the correct medication is ordered from the wholesaler and that the correct medication is received in the pharmacy
Store ENHERTU® in a different place in the fridge to other trastuzumab containing products (e.g. Herceptin® or Kadcyla®)
Ensure ENHERTU® is diluted in an infusion bag using 5% glucose solution. Do not use sodium chloride solution
Familiarise yourself with the Enhertu Summary of product Characteristics (SmPc)
Ensure that protocols to avoid medication errors are in place at the hospital/site and that they are followed
Check both the prescription and patient notes to ensure that ENHERTU® andtrastuzumab deruxtecan have been recorded as the prescribed medication
On receipt of the infusion bag, check the label on the infusion bag against the prescription and patient notes
Consider using a two nurse double‑checking system prior to infusion to ensure that the appropriate product and dosage is administered
Refer to both ENHERTU® and trastuzumab deruxtecan when discussing the drug with the patient
The maximum dose of ENHERTU® is 5.4 mg/kg once every 3 weeks
Familiarise yourself with the ENHERTU® dose modification for toxicities
Ensure ENHERTU® is diluted in an infusion bag using 5% glucose solution. Do not use sodium chloride solution.
| Overview of ENHERTU© | ||
| Trademark |  |
|
| Indication | unresectable or metastatic HER2-positive BC | |
| International Nonproprietary Name (INN) | trastuzumab deruxtecan | |
| Content of vial | 100 mg | |
| Distinctive colour | ||
| Carton image & colours | ORANGE DARK PURPLE |
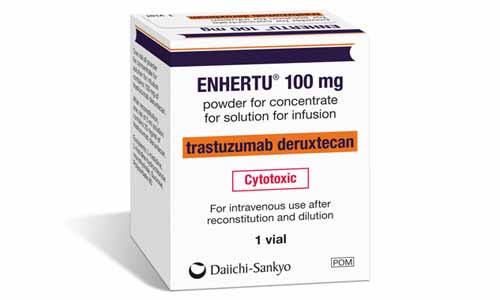 |
| Label colour | ORANGE DARK PURPLE |
 |
| Vlal colour | AMBER |  |
| Cap colour | YELLOW |  |
PATIENTS
If you have any side effects, talk to your Pharmacist, Doctor or Nurse. This includes any possible side effects not listed in the Patient Information Leaflet.
Side effects can be reported at https://aereporting.astrazeneca.com
You can also report side effects directly via <<Local Heath authority reporting information>>.
HEALTHCARE PROFESSIONALS
Reporting suspected adverse reactions after authorization of a medicine is important. It allows continual monitoring of the benefit and risk balance of the medicine to patients.
When reporting adverse reactions, please provide as much information as possible including:
Information about the patient’s medical history
Any other medicines they are taking and
Indication for Enhertu prescription.
Adverse events should be reported. Reporting forms and information can be found at <<Local Heath authority reporting information>>. Adverse Events should also be reported to AstraZeneca on 0800 783 0033 or at https://aereporting.astrazeneca.com
We Love
Receiving Feedback
Daiichi Sankyo and AstraZeneca strives to provide high-quality, user-friendly tools to support patients and healthcare prescribers in using our medicines.
Your feedback is vital in helping us to identify new and innovative ways to improve and develop these tools.
-
Saltar a la sección
- Factores del riesgo generales
- Tratamiento
